Regulatory and Clinical Development
Drug Discovery Process
The Food and Drug Administration (FDA) ensures that the medicines (drugs and biologics) and medical devices sold in this country are safe and effective

Target Identification
- Data mining using bioinformatics
- Disease Association Ex: Genomics/Proteomics, transgene, phenotyping
Target Validation
- Genetic Approaches Ex: gene editing, transgene animal knockout
- Gene expression studies Ex: Polymerase assay
- Protein expression studies Ex: IHC, Western Blot
- Pharmacological methods Ex: Chemical genomics, phenotypic screening
Hit Identification
- High-throughput screening
- Focused screening
- Virtual screening Ex: docking models
- Physiological screens
Lead Identification
Lead Optimization
- Key assays Ex: Lipophilicity, Solubility, CYP450 inhibition
Candidate Selection
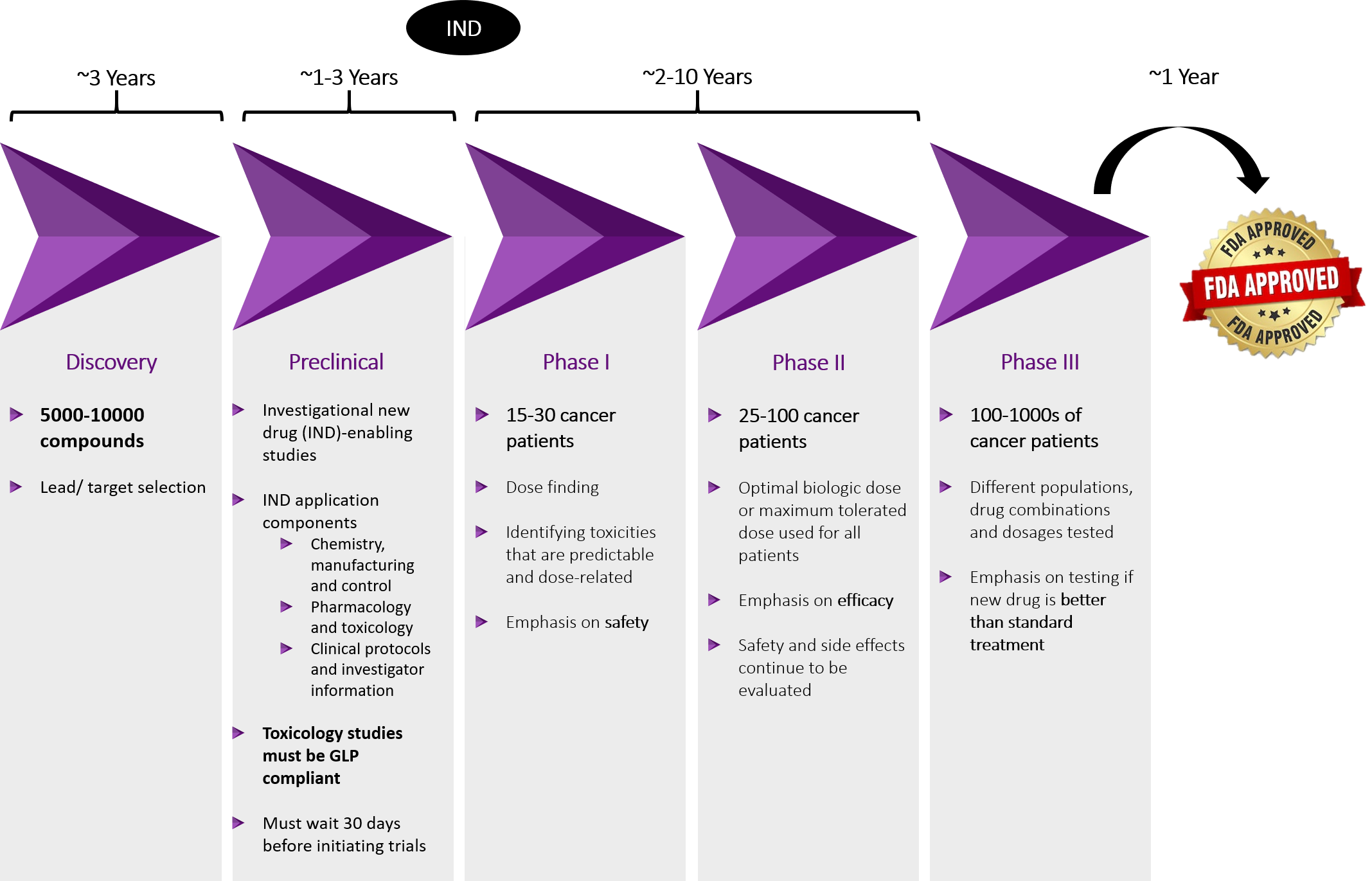
Oncology Clinical Trial Process
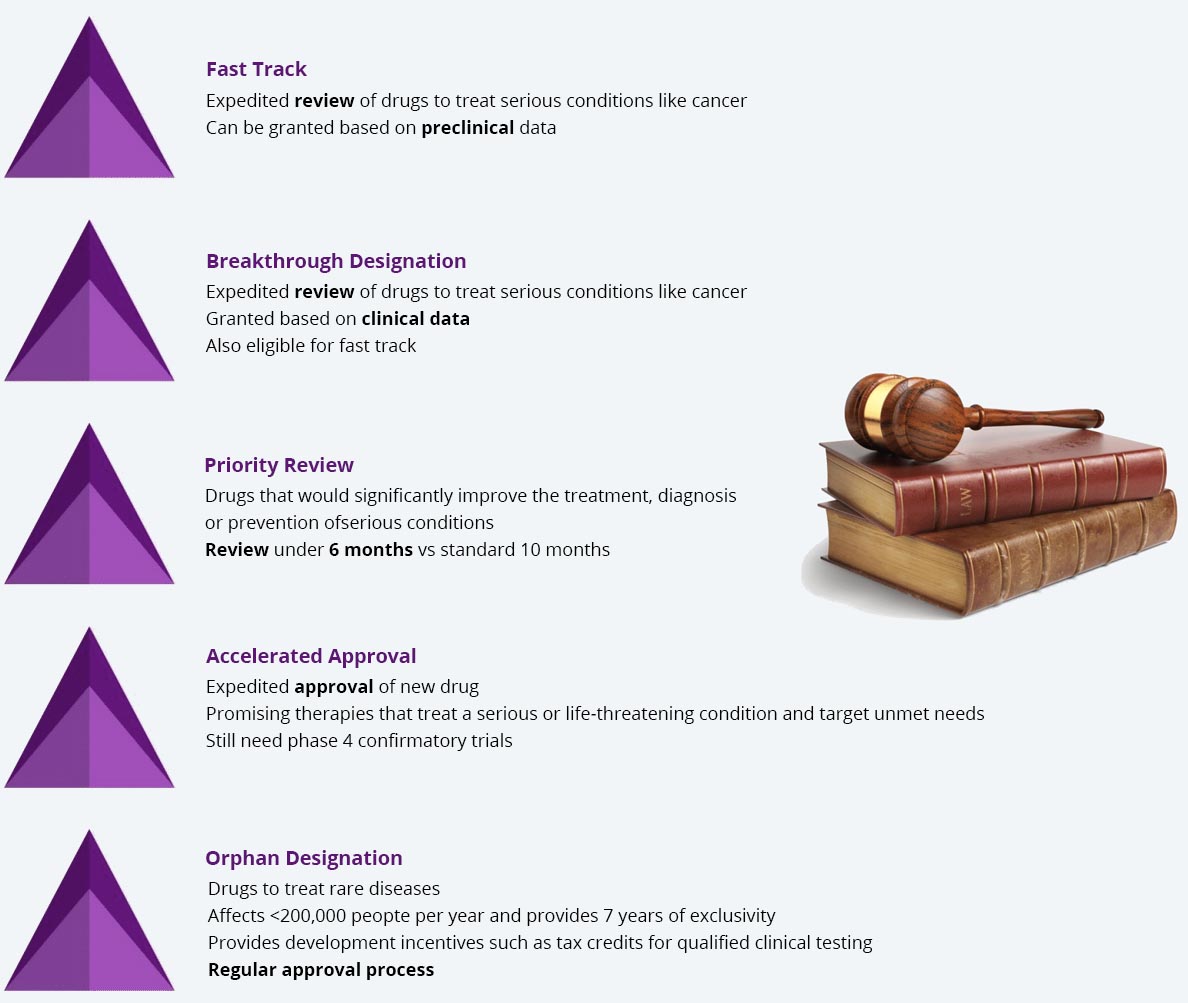
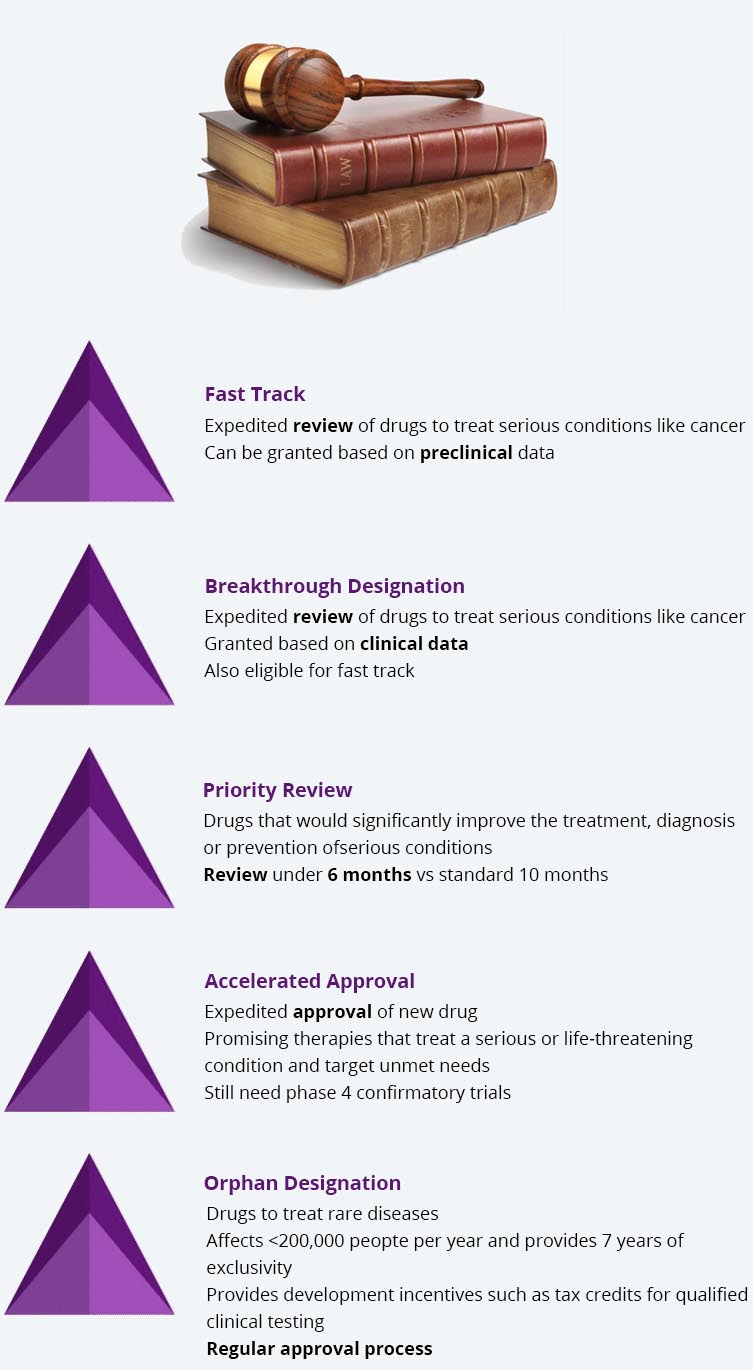
Paths to FDA Approval
Cancer qualifies for most of these pathways and multiple paths can be used to expedite FDA approval
Device
A medical device is an instrument, implant, machine, in vitro reagent that is intended for use in diagnosis of the disease or other condition OR in the cure, mitigation, treatment or prevention of disease
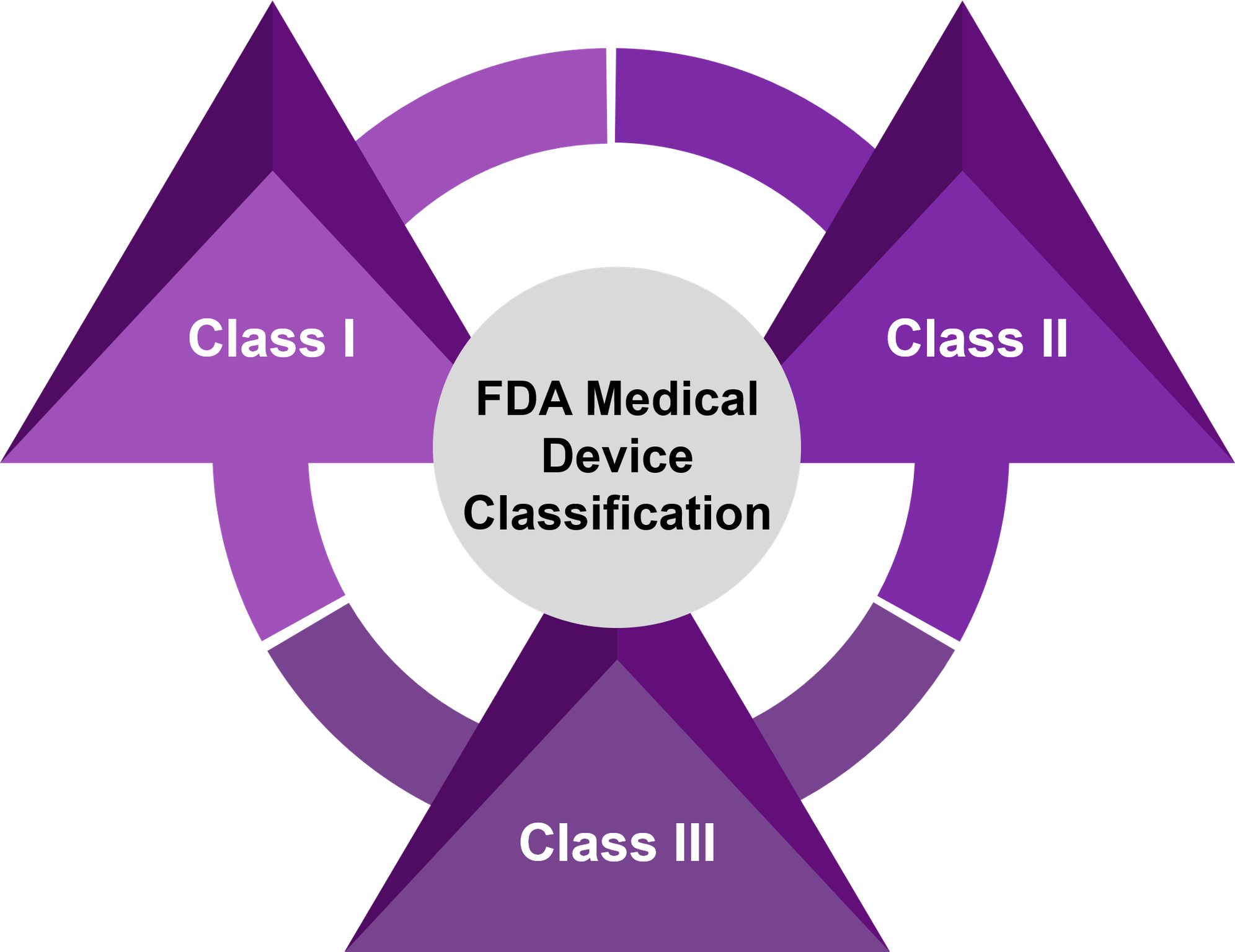
Class I
- Devices with minimal risk of harm to user
- Mostly exempt from premarket notifications 510(k) and no formal review
- No clinical studies needed
Class II
- Devices with a moderate risk of harm to the user
- Premarket notification 510(k) needed – FDA cleared
- Some may need clinical data
Class III
- Devices that sustain or support life, are implanted, or present potential high risk of illness or injury
- Premarket approval (PMA) needed – FDA approved
- Investigational Device Exemption (IDE) needed for gathering clinical data
Three different paths currently exist for obtaining Food and Drug Administration (FDA) approval of an IVD
- 510(k) – A premarket notification that allows FDA to determine whether the new device has the same intended use AND technological characteristics as a predicate device OR differences in either don’t raise questions about safety and effectiveness.
- De novo – Used for device that measures a new analyte or has an intended use for which no submission has previously been cleared. Automatically, assigns a class III designation but FDA may reclassify as class I or II if it won’t be used to make a critical medical decision concerning the diagnosis, treatment, or medical management. If class II, then 510(k) can be pursued.
- PMA – If the device does not fulfil any of the above, then the premarket approval is the pathway of choice. It is the most comprehensive and lengthy submission which contains a complete record of the studies performed to support a reasonable assurance of safety and effectiveness for use of the device, as well as information on how the device is designed and manufactured
Breakthrough Devices Program
FDA review of an application [either PMA or 510(k)] may be expedited either on request of the sponsor of a new device or based on the FDA preliminary review. To merit expedited review, the condition to be diagnosed must be life-threatening or irreversibly debilitating, and the device address an unmet medical need, as demonstrated by any one of the following: breakthrough technology, no approved alternative, significant clinically meaningful advantage, or, in the best interest of patients. The single most important determinant of review time is the quality of the submission.
If the test is done “in house,” in the designated laboratory only, for a patient sample that is sent to the laboratory from an outside facility, then the test can be potentially marketed under “home brew” guidelines (also known as laboratory developed tests) regulated under the Clinical Laboratory Improvement Amendments (CLIA).
LDT
- A laboratory developed test (LDT) is a type of IVD test that is designed, manufactured and used within a single laboratory.
- The Clinical Laboratory Improvement Amendments (CLIA) program regulates laboratories that perform testing on patient specimens in order to ensure accurate and reliable test results.
- CLIA applies when: (1) patient-specific results are reported from the laboratory to another entity; AND (2) the results are made available “for the diagnosis, prevention, or treatment of any disease or impairment of, or the assessment of the health of, human beings.”
- Tests are categorized as waived, moderate complexity or high complexity
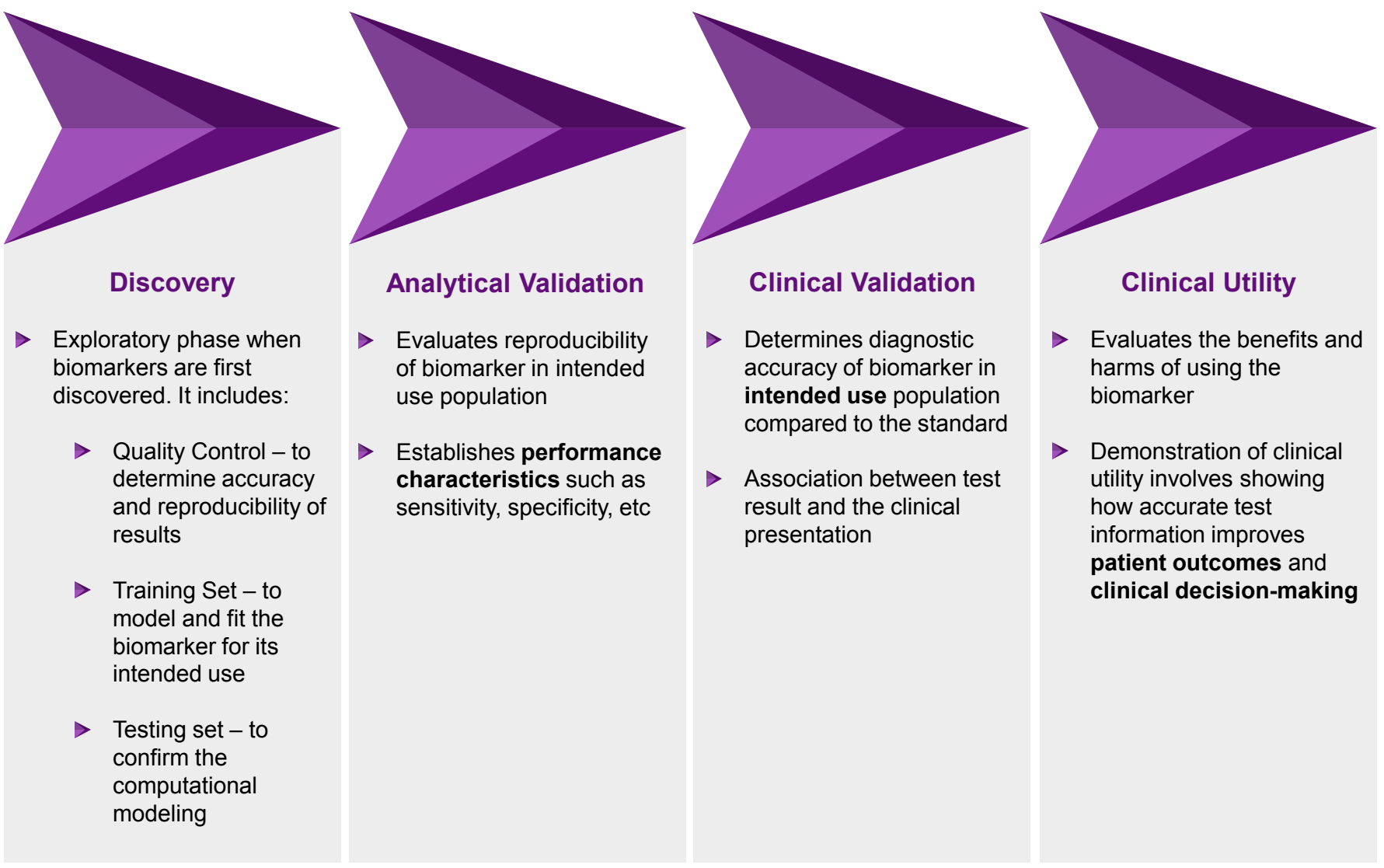
Biomarker Development
FDA vs. CLIA
- FDA’s premarket clearance and approval processes assess the analytical validity of a test system in greater depth and scope. CMS’ CLIA program DOES NOT address the clinical validity of any test, unlike FDA
- FDA’s analytical validity review is more in-depth and comprehensive than that of the CLIA program, and it is focused on the test system’s safety and effectiveness
